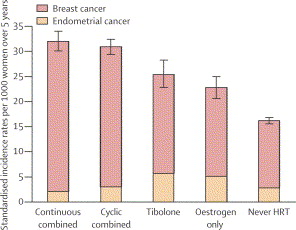
In the latest issue of the Lancet (Lancet 2003;362:414-415,419-427) a study from Great Britain was published regarding the risk of breast cancer. Over 1 million women were followed from 1996 to 2001. They were in the age group of 50 to 64. Of these 80% were postmenopausal, and these formed the basis of the study. Dr. Valerie Beral (from the Cancer Research group UK in Oxford) was the lead investigator. About half of the women were on various forms of hormone replacement therapy (HRT), the others were not and served as a control. Risks were always expressed in comparison to the controls without any hormone replacements. Here is a tabular summary of the various hormone replacement therapies and their risks of leading to breast cancer.
The relative risk of developing breast cancer did not significantly change whether HRT was taken orally, transdermally or through implanted formulations. Tibolone is a synthetic steroid used for postmenopausal symptoms and treatment of endometriosis.
Dr. Beral’s group has estimated that in Great Britain in the past 10 years about 20,000 additional cases of breast cancer were caused by HRT for menopause among women aged 50 to 64. Out of these about 75% were due to the use of the combination of estrogen/progestin.
An accompanying editorial by Dr. Chris van Weel stated that “general practitioners should discourage HRT for their patients” and, if used, should last “no longer than 3-6 months”. The investigators of this study suggested that “discontinuing HRT should be suggested in as supportive a way as possible, because no one will benefit from panic or over-reaction”.
| Findings from the British Million Women Study on HRT | |
| Detail of hormone replacement: | Breast cancer risk compared to control: |
| overall risk of HRT for all groups of HRT | 1.66-fold |
| women who stopped HRT the previous year | 1.14-fold |
| estrogen only use currently | 1.30-fold |
| estrogen-progestagen combination |
1.88-fold |
| tibolone users |
1.45-fold |
| combination HRT user less than 5 years | 1.7-fold |
| combination HRT user more than 5 years | 2.21-fold |
| equine estrogen combined with medroxyprogesterone acetate and taken at least 5 years | 2.42-fold |
| death rates from breast cancer associated with current use of HRT | 1.22-fold |
Discussion: Please keep in mind that the British authors of this study were using the drug manufactured synthetic hormone-like substances and NOT bio-identical hormones. The outcome with bio-identical hormones would have shown the opposite, namely that women would not have developed heart attacks, strokes or cancer and they would not have died prematurely. Read more about bio-identical hormone replacement in the links below.
Here is a link to a chapter on menopause from Dr. Schilling’s Net Health Book.
This link deals with bioidentical hormone replacement (see lower half of that page).
Last edited October 26, 2014