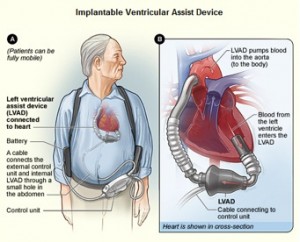
The device called HeartMate II (see image) is comparable in size and weight to a D-size battery, and it is the latest development to assist the left side of the heart. Older models were pulsating in nature, whereas the HeartMate II produces a continuous flow of blood. As a result of this, recipients of the device no longer have a discernible pulse, nor can their blood pressures be taken with the cuff around the arm. The leg muscles can naturally produce a surrogate pulse, and in the three years of human testing there have not been any problems related to the lack of pulse. It has been implanted at selected American test sites, and recently a 65 year old male patient has become the first Canadian to be implanted with the device at McGill University Health Center. Dr. Renzo Cecere, the heart surgeon involved, is very enthusiastic with the outcome. The patient made an exemplary recovery and stated that he feels more alive than he has in years, which is impressive, as in the past he could hardly take a step. He suffered of end stage left ventricular failure, and at this point only patients with this condition can enroll in the HeartMate II clinical trials.
Dr.Cecere foresees the device being appropriate for many Canadians. The longevity of the “mechanical heart” (it is good for 10 years) will make it a true alternative to a heart transplant. Some patients cannot receive a transplant because of age or medical conditions. Patients with a history of cancer would be the ones who could not be treated successfully with a heart transplant. The anti-rejection drugs that have to be taken on an ongoing basis produce immunosuppression, and this can revive a cancer in remission.
So far the biggest known risk factors are bleeding, as patients have to take small amounts of blood thinners. Another risk is infection.
At this point the cost for the HeartMate II amounts to about $100, 000, and it does not have the Health Canada approval for general use yet.
More information about congestive heart failure: http://nethealthbook.com/cardiovascular-disease/heart-disease/congestive-heart-failure/
Reference: National Review of Medicine, January 15, 2007, page 36-37
Comment Nov. 15, 2012: The device was approved by the FDA in April of 2008.
Last edited November 2, 2014