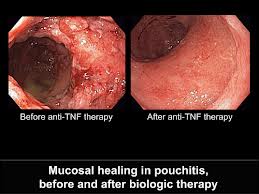
So far the strategy of treating patients, who were newly diagnosed with Crohn’s disease, was the use of corticosteroids to control abdominal pain and bloody diarrhea. The conventional mode of treatment consisted of a “step-up” treatment: after corticosteroids the use of immunosuppressants and finally antibody treatment would follow to curb the inflammatory response. International data which were published in the Lancet (February 23 issue) points to a safer and more effective treatment protocol. It consists of a “top-down” approach rather than of the conventional “step-up” approach. Patients receive immunosuppression early in the form of azathioprine and also the antibody infliximab, known as Remicade. Another study examined patients who were on maintenance therapy with adalimumab (Humira). They experienced sustained improvements in the symptoms that are associated with Crohn’s disease. After 4 weeks of induction therapy with the medication patients with mild and severe depression had improved to such an extent that they returned to the normal range. They were also assessed regarding fatigue and after treatment they showed a significant improvement in their daily functioning. The two treatment protocols were compared in a trial involving 129 patients with Crohn’s disease who had no previous treatment. At the end of the trial the researcher found that 65% of the group that had received the “top-down” treatment was symptom free after 26 weeks of treatment. Contrary to this only 36% of the “step-up” patients went into remission during the same time.
When the patients were examined after 1 year, 62% of the “top-down” group was still symptom free, but only 42% of the “step-up” group had no symptoms. Dr. Brian Feagan of the University of Western Ontario coordinated this trial involving international sites in Belgium, Holland and Germany. He points out that the newly diagnosed Crohn’s disease patient that has the worst prognostic signs will benefit from this form of treatment. The top-down modality also is safer, as it protects the patient from high exposure to steroids. Similar results were demonstrated in patients with rheumatoid arthritis. The results of these trials and Dr. Feagan’s research suggest that the top-down treatment option could also give the best chance to patients with other chronic autoimmune diseases such as ulcerative colitis.
More information about Crohn’s disease: http://nethealthbook.com/digestive-system-and-gastrointestinal-disorders/crohns-disease-crohns-disease/
Reference: The Medical Post, March 4, 2008, page 2; April 1, 2008, page 17
Last edited November 3, 2014