A recent publication reported about a new immunotherapy approach against cancer. The model it dealt with was a very vicious brain cancer with the name glioblastoma. The results of this research were subsequently transferred to another vicious cancer, osteosarcoma, which is a form of bone cancer with a very poor prognosis. Researchers have to do further clinical experiments to establish this new immunotherapy in osteosarcoma patients. Physicians completed the following experiments and clinical studies.
Oncolytic virus Delta-24-RGD can lead to remission in glioblastoma patients
Researchers at the University of Navarra, Pamplona, Spain together with The University of Texas MD Anderson Cancer Center in the US investigated glioblastoma patients. They found that treatment of glioblastoma patients with oncolytic viruses Delta-24-RGD led to a greater than 3-year remission in 20% of cases. Normally, patients with a glioblastoma survive only 9 months on average. 12% had a greater than 95% reduction in the size of the tumor. This was a phase 1 clinical study with 37 patients who had recurrent malignant glioblastoma. The authors said: “Oncolytic adenoviruses are attractive therapeutic agents because they can kill tumor stem cells and induce cell death by several mechanisms, including direct lysis, expression of toxic proteins, induction of cytokines, and T-cell–mediated immunity.” The particular oncogenic virus that the researchers used was an adenovirus Delta-24-RGD.
Transferring glioblastoma results to a cure for osteosarcoma
The same researchers wanted to see whether the cure rates of treating patients with glioblastoma was transferable to other cancer patients. In particular they were interested in patients with osteosarcoma, which is a similarly vicious cancer. Advanced osteosarcoma has a survival rate of 27% after 5 years. The researchers first did experiments with a human osteosarcoma cell line in tissue culture and at the same time a murine osteosarcoma cell line. Later they tested the action of oncolytic viruses Delta-24-RGD in a mouse model.
Experiments with osteosarcoma cells in tissue culture
The advantage of such experiments is that you can control all the parameters easily in a Petri dish. But critics say that this is far removed from osteosarcoma behavior in humans. Researchers found that the oncolytic virus Delta-24-RGD killed many osteosarcoma cells in vitro. They also were able to insert a new gene into the oncolytic virus, which was equally effective in killing osteosarcoma cells. They called this virus Delta-24-ACT.
Curing osteosarcoma in a mouse model
Next the researchers tested effectiveness of the oncolytic viruses, Delta-24-ACT and Delta-24-RDG in mice. They injected osteosarcoma cells from tissue culture into the tibia of mice. Tumor growth was subsequently measured. The experimental groups were given two oncolytic virus infections, the control group did not. On day 10 and 18 the researchers could see that controls had faster growing tumors compared to the experimental groups. The experimental groups had less tumor side effects. And the experimental mice survived longer than the controls. Further research showed that the oncolytic viruses produced a 4-1BBL protein, which stimulated the animals’ immune system to fight the osteosarcoma.
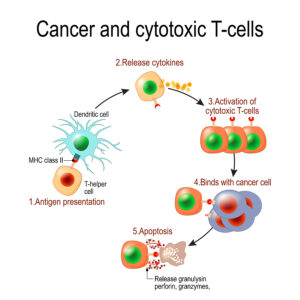
New immunotherapy approach against cancer: Effector T cells
Researchers could prove that in mice treated with oncolytic viruses it was the special protein (4-1BBL) that stimulated T lymphocytes to become killer T cells. They in turn attacked the osteosarcoma cells.
New immunotherapy approach against cancer: The need for human research
Doing research in humans is more complicated than in a mouse model. But in order to improve survival rates in patients with osteosarcoma human research is absolutely essential. However, research is complex and the effects of oncolytic viruses is only in the 20% range with regard to increasing survival. This requires more research. It may be that instead of oncolytic viruses a stimulatory protein would arm T cells to become killer T cells that fight the cancer.
Conclusion
Glioblastoma patients had a better survival after treatment with oncolytic viruses Delta-24-RGD. Researchers translated this type of research to another cancer, osteosarcoma. This also has a poor prognosis, Researchers did experiments in tissue culture and in a mouse model. They were able to show that oncolytic viruses produced a 4-1BBL protein, which stimulated the animals’ immune system to fight the osteosarcoma. Specifically, the protein armed T lymphocytes and turned them into killer T lymphocytes. These destroyed osteosarcoma cells in tissue culture or in the mouse model. It is encouraging to see positive results in a laboratory setting of a tissue culture. The step further in an animal experiment is also a positive achievement. More research will improve the cure rates of osteosarcoma. The effective treatment of osteosarcoma in humans is still far away! The next step is human research that shows improvements in patients’ survival rates.